

Clinical Study Report (CSR) is lengthy and manual document which discusses about the Clinical Trials methods and results. Creating this document consumes more manual effort. Scientific documents such as Study Protocol, Statistical Analysis Plan (SAP), Case Report Form (CRF), Safety Narratives, In-Text Tables, Tables, Listings and Figures (TLFs) constitute considerable amount of information to the CSR.
The tool automatically collects and collates information from sources such as Protocol, SAP etc., in the appropriate sections as per ICH E3 guidelines for CSR document. The tool automates significant manual efforts of the medical writers. The tool also has the workflow module which can be configured as per the sponsor needs.
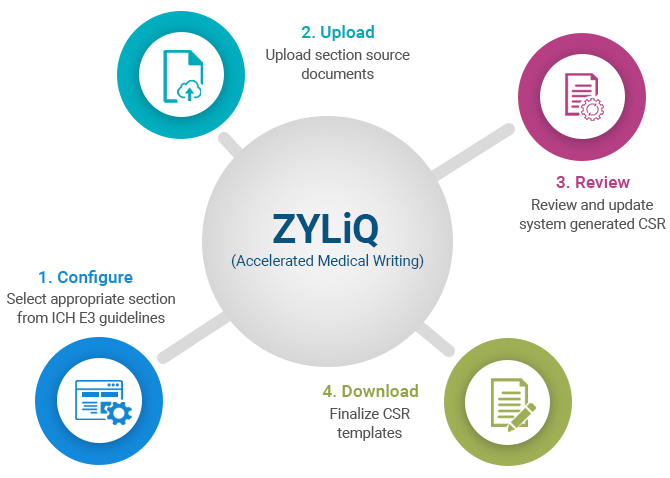
The system is designed to generate pre-filled CSR with information from Protocol, SAP and other sources as per ICH-E3 guidelines in the respective sections which could save medical writers 60%-70% of time.
Medical writers can focus more on their interpretation of study results and their discussion points.
In workflow integration, System can configure based on the sponsors needs.